Spectra!
Visible emission line spectra of some elements and light sources. (latest
additions 7/4/99.)
Skip to Other Spectra Sites
Please beware that reproduction accuracy is limited. In order to maximize speed
and compatibility with various graphical web browsers, I have largely limited
myself to the 216 colors used by an old version of Netscape running under an old
version of Windows with 8 bit video. 125 of these colors have nonzero use of
all three primaries, about 12-15 are obviously non-spectral magentas and
purples, and and a few others are questionable purplish colors. That leaves
only about 75 colors to use, including bright and dark shades.
In addition, computer monitors are not capable of accurately reproducing
spectral colors, especially not deep red, deep green, and deep cyan.
This way to the GIF file alone!
Explanations
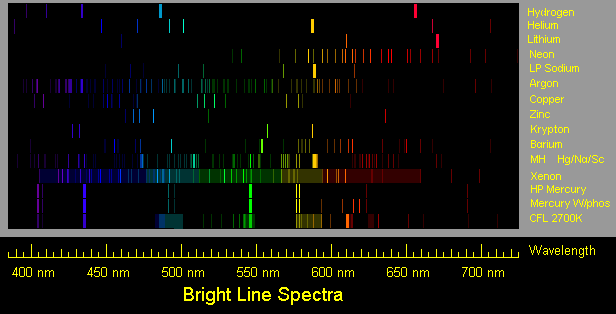
First spectrum is hydrogen, typical of a hydrogen spectrum tube.
Second spectrum is helium, typical of a helium spectrum tube.
Third spectrum is lithium, as typically from a flame or an electric arc.
Fourth spectrum is neon.
Fifth spectrum is low pressure sodium, but with secondary lines exaggerated.
Sixth spectrum is argon, typical of an argon glow lamp or spectrum tube.
Seventh spectrum is copper, drawn using a wavelength table and Ioannis
Galidakis' photos of a copper arc spectrum (see link below). Oxide
lines which may appear in the flame spectrum are not shown.
Eighth spectrum is zinc, drawn using a wavelength table and a photo by
Ioannis Galidakis of a zinc arc spectrum. Intensity of the red line is
shown for the slightly greenish light blue usual zinc arc, but Ioannis
reports getting a pinkish zinc arc and shows the red line to be brighter.
Ninth spectrum is barium. Oxide lines are not included.
Tenth spectrum is krypton. Ion lines typical of flashlamp use are not
included.
11th spectrum is that of the most common North American variety of a metal
halide lamp, which is basically a mercury vapor lamp enhanced with iodides of
sodium and scandium.
12th spectrum is that of a xenon flashtube, operated with a higher than usual
voltage and a lower than usual energy level to favor a line spectrum. An
actual typical xenon spectrum generally has a strong continuous spectrum, which
I show more dimly than usually actually occurs in order to show the lines. The
lines are mainly those of excited xenon ions, rather than excited neutral xenon
atoms. At lower current, the most distinct visible spectral lines are two close
together in the blue and the brightness is usually low.
13th spectrum is high pressure mercury vapor, typical of a mercury vapor lamp.
Low pressure mercury vapor has a similar spectrum, except the green line is
slightly dimmer and the yellow lines are significantly dimmer.
14th spectrum is a mercury lamp with the common Deluxe White phosphor.
15th spectrum is a compact fluorescent lamp of the 2700K color.
The spectra site of
I. N. Galidakis, with spectra photos taken using an Amici prism.
National
Institute of Standards and Technology spectra tables top page.
National
Institute of Standards and Technology "Handbook of Basic Atomic Spectra".
Written by Don Klipstein.
Please read my Copyright and authorship info.
The above spectra artwork is copyright (C) Donald L. Klipstein 1997,
1998, 1999. Distribution of copies mentioning the copyright claim and
authorship is permitted.
Please read my Disclaimer.
